The COVID-19 Coronavirus has had a once-in-a-generation type of impact on our lives. Its devasatation has reached far and wide; whether it’s our jobs, our day-to-day lives, or even loved ones. The virus remains an enigma - some individuals seem to suffer more than others, and the virus' behaviour seems to be more and more unpredictable as the days go by. One strategy that has been hailed as an effective method to manage the crisis is testing: finding those who have been infected with the virus. Here in the UK, the government has recently turned to getting tests from Roche.
These diagnostic tests from Roche are serology tests: they take a sample of an individual’s blood, and test for antibodies. Hold on a minute!
- What exactly are antibodies?
- What does having antibodies mean?
- How does this help with testing for COVID-19?
In this post, I’ll do a little primer on what antibodies are, and hopefully show you why they are so cool.
If you have…
- 30 seconds: They are Y-shaped proteins that can recognise foreign molecules. Good antibodies lead to the rest of your immune system helping to clear out that foreign molecule.
- 10 minutes: Go on.
NB: Unlike my other posts this one will be fairly biology heavy, you’ve been warned. I’ll have a jargon buster session at the end.
The antibody molecule
Antibodies are proteins. In humans, they look like the letter Y, as shown below:
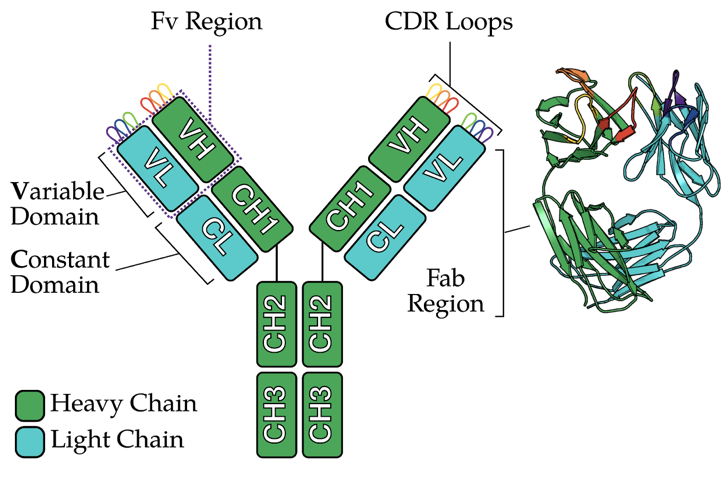
The antibody molecule has two pairs of two protein chains:
- Two “heavy” chains (green)
- Two “light” chains (cyan)
Each chain can be sub-divided into globular units, or domains. A single chain can have:
- A variable domain (VH, VL)
- A constant domain (CH, CL)
The two variable domains from each chain (VH+VL) form the Fv region. Within the Fv are six loops, known as the CDR loops.
This is key: the amino acid sequence (and thus the shape) of the CDR loops determines whether an antibody can bind things, e.g. the COVID-19 virus, with sufficient strength. Put another way, I like to think of the entire Fv region as being similar to our hands, and the CDR loops are like our fingers. Depending (largely) on the shape of your fingers, you can hold on to different sized objects.
For example, this is the molecular structure of an antibody binding its target molecule, also known as the antigen:
Notice that the pink blob (antigen) is in close contact with the CDR loops (coloured red, yellow, purple…). Furthermore, as the antibody has two Fv regions, it can bind to two antigens simultaneously. One more thing to mention here is that the combined set of CDR loops recognise a particular spot on the antigen, known as the epitope. This means that, in theory, that pink blob can be recognised by lots of different antibodies throughout its entire surface.
The antibody’s origins
Antibodies are synthesised from a type of white blood cell called the B-lymphocyte. To cut a couple weeks' and textbook chapters short, every person has billions of B-cells, each of which produces one antibody. Each B-cell performs a process known as V(D)J recombination to randomly stitch together parts of the genome to produce an antibody:
To start with, an antibody starts its life as a protein on the surface of the B-cell (technically called a B-cell receptor). When a circulating B-cell comes in contact with its target antigen, the B-cell converts the B-cell receptor to a soluble form that circulates in the bloodstream. This entire process takes a few weeks after the initial infection.
Altogether, if COVID-19 binding antibodies are detected in an individual, one can assume that they have been infected with the virus a couple of weeks prior to the time of taking a test.
What do antibodies do again?
Basically antibodies bind things. What’s important is what happens afterward. Antibodies trigger a series of responses from the immune system after recognising the antigen.
Broadly speaking, in all three cases, the antibody acts as a “flag” that tells other parts of the immune response to do something about the antigen. Against viruses, antibodies can also stop them from exiting an infected human cell. In fact, the range of “healthy” responses mediated by antibodies is discussed in this paper by Tay et al.
Are all antibodies equal? Not quite. In some unfortunate cases, antibodies might facilitate viral entry, as shown in panel B below:
This phenomenon, known as antibody-directed enhancement, has been documented in that paper by Tay et al. that I described above. Other reviews like this one by de Alwis et al speculate that antibodies can cause hyper-active immune responses, which can also be detrimental.
Then how do these antibody tests work?
Let’s recap everything discussed above:
- Antibodies have two pairs of heavy-light chain pairs, leading to two identical binding sites (Fv regions)
- An antibody comes from a B-cell after infection, and is then released to the bloodstream
- Antibodies can trigger immune responses in individuals, but sometimes those antibodies may not be so useful
Diagnostic tests like those from Roche leverage the first two points. It looks to see if a patient has developed antibodies against COVID-19 (whether it’s protective or not). This is done by a “double antigen sandwich”:
Essentially, the test counts on the fact that a single antibody molecule can bind two antigens - one on each Fv. Each antigen is either:
- Hooked up to a biotin molecule (which acts as an anchor to hold antibodies in place), or
- It is ruthenylated, making the antigen “light up” in the testing kit
I’m deliberately skipping a lot of the details of the test, but these are the basic biological details. While the test provides valuable information for whether or not you have antibodies against COVID-19, the test does not confirm whether you are immune to the virus. To confirm this, other tests would be necessary. For example, antibodies would have to be isolated and tested to see if they can neutralise the virus in a separate lab experiment.
Wrap-up
I think what is extremely remarkable about antibodies is that they are natural, in-built defence mechanisms. Almost magically, they can protect us from viruses that we have never seen before, like COVID-19. By understanding their biological structure and mechanism, this has allowed us to develop instrumental tools like Roche’s diagnostic tests.
This post was intended to be a basic primer that (hopefully) landed somewhere between a Guardian article and a journal article (so, a textbook I guess?) There is still so much more we can talk about in this space, whether it’s:
- How do we design external antibodies to fight the virus?
- What makes antibodies go bad?
- How can artificial intelligence approaches alongside antibodies help to fight COVID-19?
But perhaps that’s for another time.
Acknowledgements / References
For any uncited figures, they are almost all from my PhD thesis. The exception is the picture of an antibody binding two pink blobs, which I made for a presentation.
Otherwise I’ve referred to the figures directly and I’m incredibly grateful for scientific illustrators and creators at NPG and Roche. Good illustrations go a long way to helping everyone understand how things work!